
It may share electrons with an adjoining atom to make a covalent bond, or it could take one electron away to type an ionic bond. As a result, halogens are probably the most reactive nonmetals, as they solely require one electron to form bonds. To create a covalent hyperlink, they either remove an electron from another atom or share an electron from another storm. Because the valence electrons are at progressively greater energies in groups, the nonmetal’s reactivity reduces as a end result of the atoms are unable to achieve stability by acquiring electrons. The internal transition metals are the weather in the two rows which are most often shown below the the rest of the opposite parts within the periodic desk. It is most secure to remember that the inner transition metals have three valence electrons, but as is often the case, there are a few exceptions to that rule.
For impartial atoms, the number of valence electrons is the same as the atom’s major group number. Formula to calculate valence electrons for impartial atoms. This additionally signifies that when looking at a bunch number, exclude the transition metals. They are positioned in the block in the center of the periodic table.
The electrons revolving within the outer most orbit of a atom has highest vitality degree. Valence electrons are loosely attached to the nucleus. Therefore, less amount of power is required to drag an electron from the outer most orbits.
For these compounds, the distinction between the electronegativities of the weather is large enough to be significant, however not massive sufficient to classify the compound as ionic. When a pair of isolated hydrogen atoms are introduced together, two new forces of attraction appear due to the attraction between the electron on one atom and the proton on the opposite. An isolated hydrogen atom incorporates one proton and one electron held collectively by the drive of attraction between oppositely charged particles. The magnitude of this force is the same as the product of the charge on the electron times the cost on the proton divided by the square of the distance between these particles . -is best supported by the knowledge in the chart. This exercise ought to be accomplished as a person activity.
That is, the iron atom has a complete of twenty-six electrons. While the variety of valence electrons will increase by one over time, the variety of shells stays fixed. The variety of shells around an element’s nucleus is indicated by the period number .
At some point the columns could be lightly shaded and a legend could presumably be added. This works greatest when both all the information is on the table or when discussing properties. A cathode ray tube is a glass tube with two electrodes which may be related to a power hur många valenselektroner har kol supply supplying electricity. The anode has a small hole in order that the rays can cross via. A phosphor coating on the opposite end of the tube glows when the cathode rays strike it. Gases which don’t mix with other parts to form compounds.
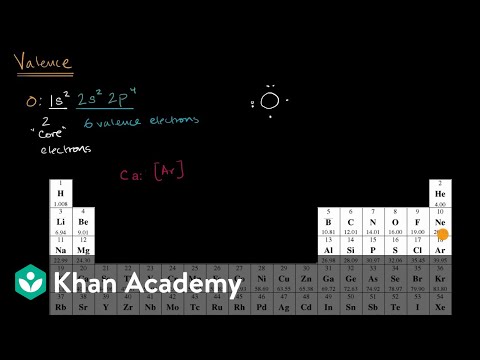
From the electron configuration of oxygen atoms, we see that the second orbit of oxygen is the last shell and there are a complete of six electrons in the last orbit. So, we are able to easily say that the oxygen atom has six valence electrons. However, if we contemplate the transition metals (groups \(3 – 12\)), discovering the valence electron is kind of difficult. The transition parts have a \(\)-subshell which plays a significant position in determining the electronic configuration of those elements. Oxygen will acquire 2 more valence electrons, to realize a secure Neon fuel electron configuration of eight outermost electrons.
The inner transition parts have incomplete f-subshells and they are very near the outer s-subshell. Hence, for inside transition parts, the electrons of each f-subshells as well as s-subshell behave like valence electrons. In some internal transition metals, the electrons of incomplete d-orbitals are additionally thought of as valence electrons. The electron configuration of mercury can be used to discover out the variety of valence electrons. The electron configuration is written as 1s2 2s2p6 3s2p6d10 4s2p6d10f14 5s2p6d10 6s2.
Electrons are distributed in numerous power shells denoted by \(\,\,\,…\) generally identified as the electronic configuration of the element. By figuring out the electronic configuration of a component, we will determine the number of valence electrons present in the atom’s valence shell. The electrons spend most of their time on the chlorine atom. In this blog we will decide the number of valence electrons in phosphorus atom. This weblog will present you detailed information about phosphorus and it’s valence electrons.
The last shell of a component has 1, 2 or three electrons, those parts are known as metals. In chemistry, valence electrons are the electrons which might be located within the outermost electron shell of a component. This is not true, as a end result of the transition metals can easily lose electrons from their d orbitals as properly as the outer s orbitals. This query cannot be answered without contemplating the orbital electronic configurations of the elements. Noble gases have full orbitals and are, thus, nonreactive, giving them the identical traits as core electrons – low energy and a lack of a need to bond. Therefore, we are able to outline all elements’ core electrons according to their earlier noble fuel.
Be positive to know when to add or subtract from the last orbital for finding valence electrons. Noble gases have eight valence electrons – the most secure state for an element. When the variety of electrons in an atom’s outermost shell approaches its most capacity, valency is determined differently. The outermost shell of the fluorine atom possesses seven electrons, and its valency could be seven, but it is easier for it to achieve one electron than to lose seven.